Adding Complementary Longevity Treatments [Survey]
MedEsthetics
AUGUST 7, 2024
Are you considering adding complementary longevity treatments to your services (hormone treatments/anti-aging supplements)? Answer our 1-question survey and let us know!

MedEsthetics
AUGUST 7, 2024
Are you considering adding complementary longevity treatments to your services (hormone treatments/anti-aging supplements)? Answer our 1-question survey and let us know!

Salon Today
AUGUST 7, 2024
A marketing expert launches a separate website to drive new clients to the salon's extension services and explains how SEO is growing this category for them.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Alison Bladh
AUGUST 7, 2024
Scrambled Eggs with Smoked Salmon (with a Plant-Based Option) Menopause fatigue can be challenging, but a nutritious breakfast can make a significant difference. This Scrambled Eggs with Smoked Salmon recipe is designed to provide the energy and nutrients needed to start your day right. I've also included a plant-based option to cater to diverse dietary preferences.

Beauty Launchpad
AUGUST 7, 2024
NAHA is the most prestigious professional beauty competition in North America, celebrating the creativity and skill of the salon industry’s top artists.
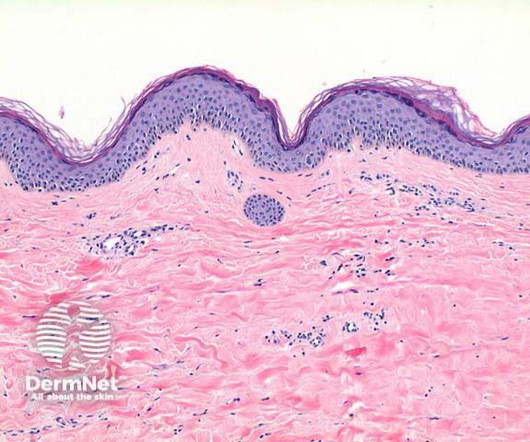
Dermatology Times
AUGUST 7, 2024
Topical corticosteroids are the gold standard treatment for VLS, yet women with skin of color are not represented in studies.

Salon Today
AUGUST 7, 2024
While maintaining a fitness routine can be challenging anywhere, living in a city that supports an active lifestyle can make a significant difference. A new study names the best cities for an active lifestyle.
Esthetician Leadership Today brings together the best content for skin care professionals from the widest variety of industry thought leaders.

Dermatology Times
AUGUST 7, 2024
Our August issue covers common back-to-school skin conditions, psoriasis research, OX40 inhibition for atopic dermatitis, and pediatric dermatology meeting highlights.

Salon Today
AUGUST 7, 2024
Nathalie De Gouveia has been appointed to lead Wella Company’s largest market.

AAAMS
AUGUST 7, 2024
Worldwide, medical aesthetics careers are growing in demand, thanks to innovation in the available products and services and increased awareness and acceptance on social media. Discover the most promising jobs in this field, how to get them and what you need to jump-start a career as a beauty and health care provider. What Medical Aesthetics Entails Medical aesthetics involves enhancing patients’ appearance using noninvasive, elective treatments such as dermal fillers, laser treatments and

Salon Today
AUGUST 7, 2024
The Makeup Show returns to Chicago November 16-17, bringing its intimate experience to the professional beauty community.
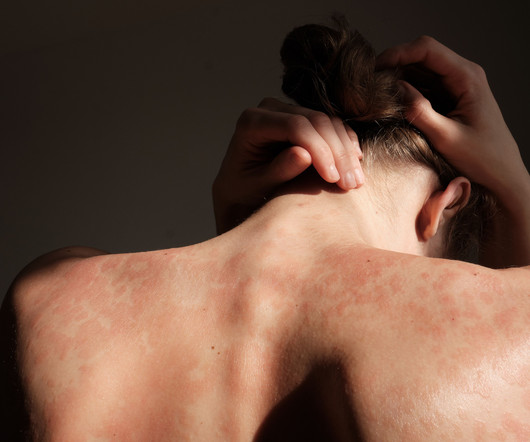
Dermatology Times
AUGUST 7, 2024
Both 8-week and 16-week dosing intervals of guselkumab achieved similar clinical outcomes and patient-reported responses in the GUIDE trial.

The Dermatology Digest
AUGUST 7, 2024
The U.S. Food Drug Administration (FDA) approved Alembic’s generic acitretin formulation for severe psoriasis. Acitretin is an oral retinoid. The company announced final approval from the FDA for its Abbreviated New Drug Application (ANDA) Acitretin Capsules USP, 10 mg, 17.5 mg, and 25 mg. The approved ANDA is therapeutically equivalent to the reference listed drug product (RLD), Soriatane Capsules, 10 mg, 17.5 mg, and 25 mg, of Stiefel Laboratories, Inc.
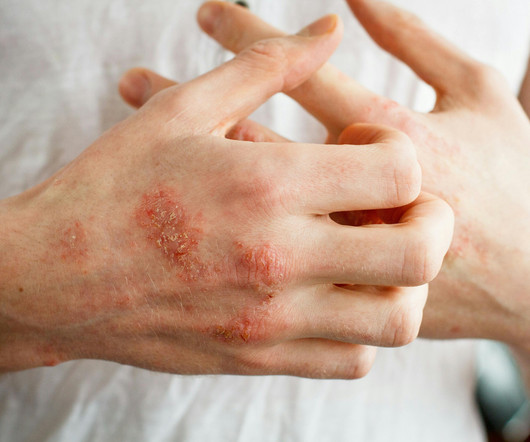
Dermatology Times
AUGUST 7, 2024
Researchers said they hope this consensus guides prescribing decisions among clinicians and encourages development of a standard approach.

Dermatology Times
AUGUST 7, 2024
The findings highlight the need for diverse genetic studies to understand their broader impact on autoimmune diseases.

Dermatology Times
AUGUST 7, 2024
A group of international experts focused on the technology’s potential to standardize and enhance patient assessments.
Let's personalize your content