Maui Derm News: Delgocitinib Cream Bests Alitretinoin in Phase 3 CHE Trial
The Dermatology Digest
JANUARY 29, 2025
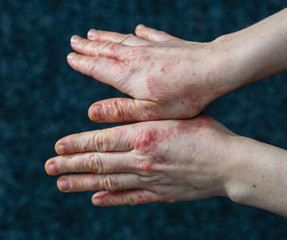
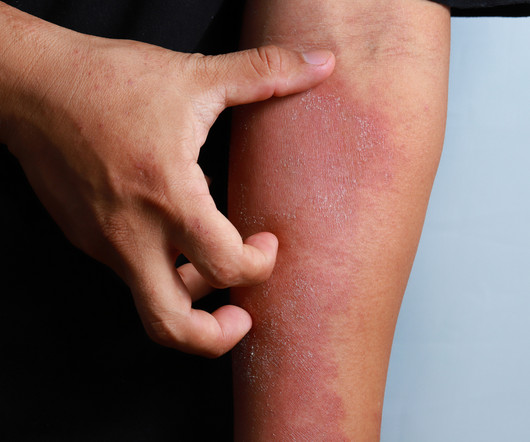
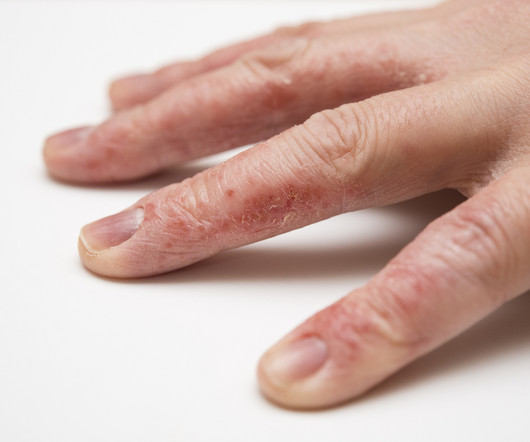
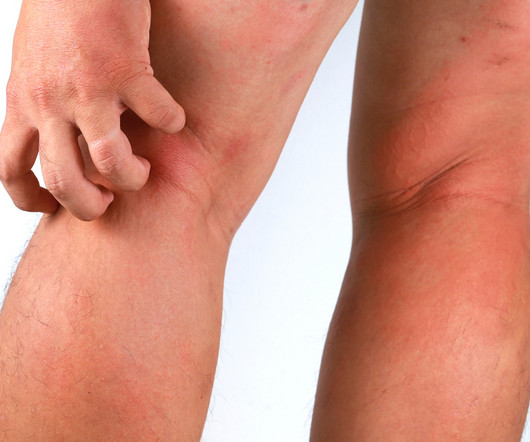
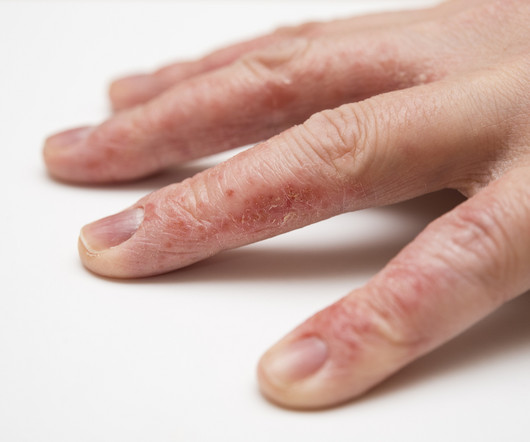
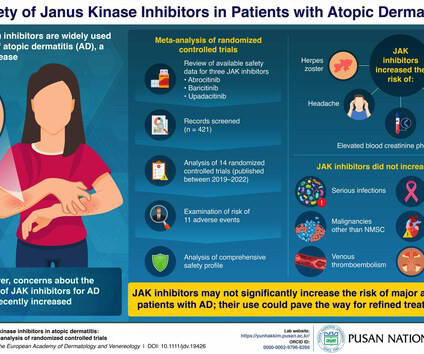
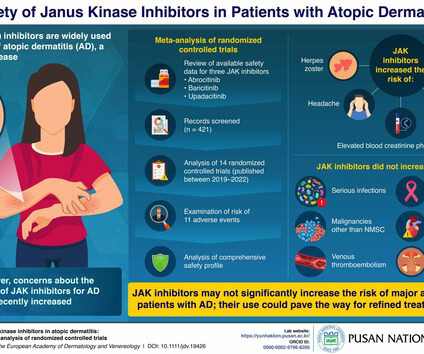
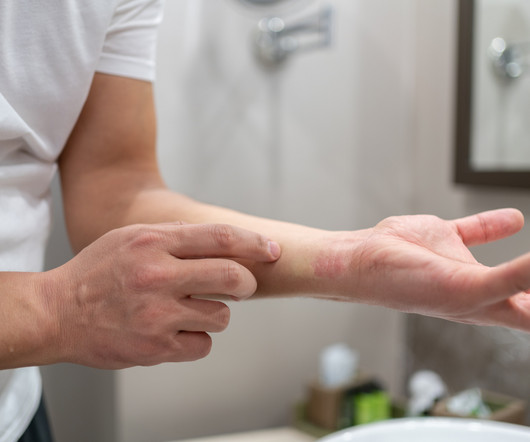
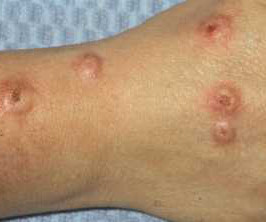
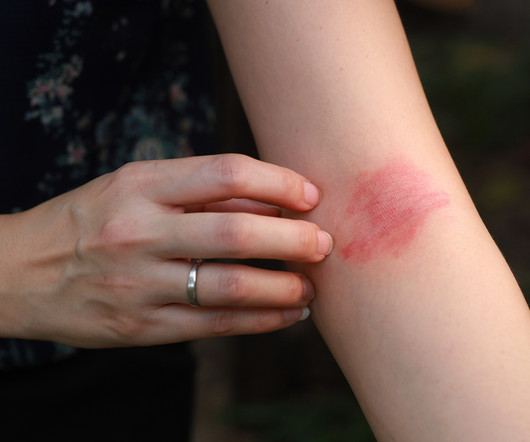
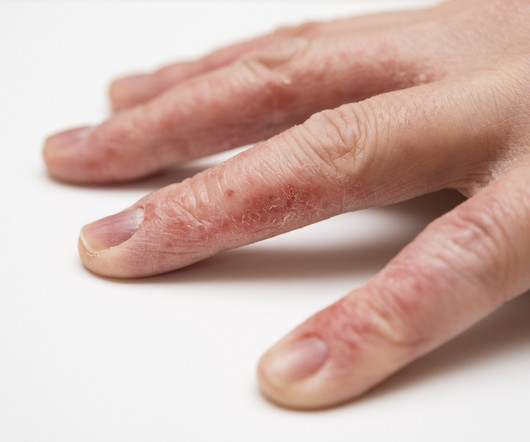
Delgocitinib cream 20mg/g bested oral alitretinoin for the treatment of chronic hand eczema, according to the Phase 3 DELTA FORCE trial. The topical pan-Janus kinase (JAK) inhibitor demonstrated superior treatment effects, quality of life improvements, and a more favorable safety profile compared to oral alitretinoin over 24 weeks.

































Let's personalize your content