Rosacea Pipeline Watch: FDA Accepts Journey Medical’s NDA for DFD-29
The Dermatology Digest
MARCH 18, 2024
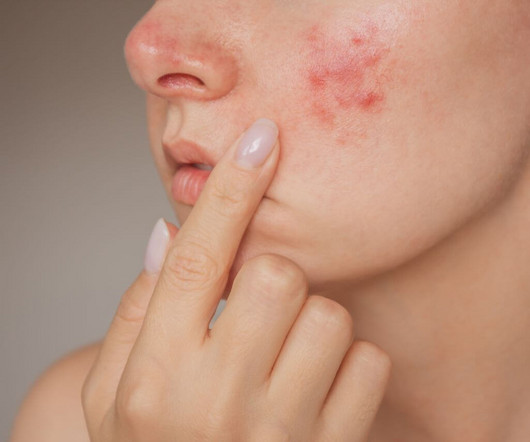
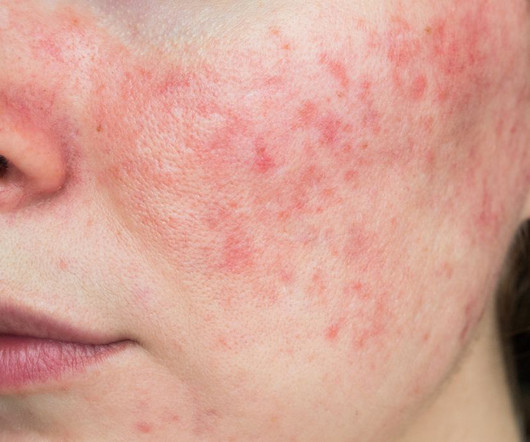
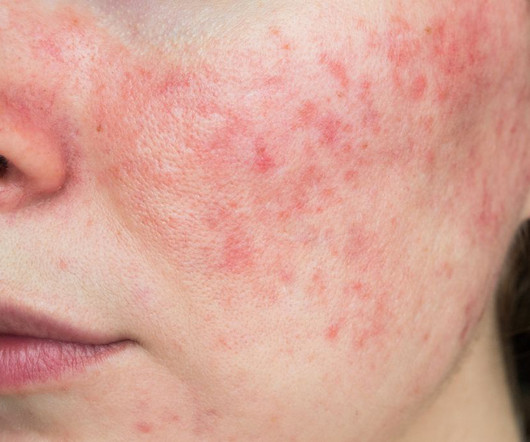
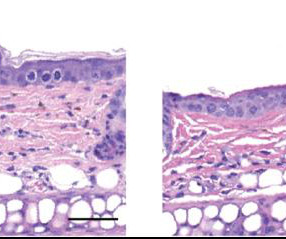
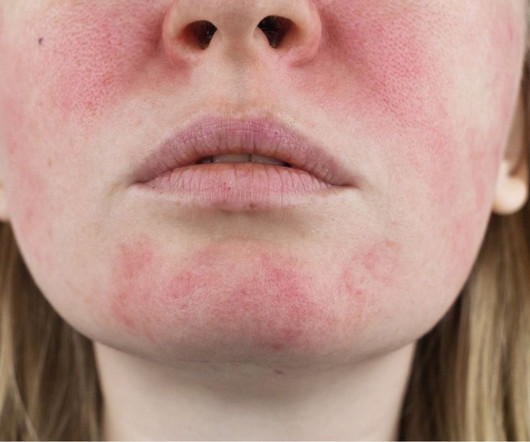
Food and Drug Administration (FDA) has accepted Journey Medical’s New Drug Application (NDA) for DFD-29 (Minocycline Hydrochloride Modified Release Capsules, 40 mg) for the treatment of inflammatory lesions and erythema of rosacea in adults. The FDA has set a Prescription Drug User Fee Act (PDUFA) goal date of November 4, 2024. “We










































Let's personalize your content