New Ladder Helps Guide Choice of Non-steroidal Treatments in AD
The Dermatology Digest
APRIL 7, 2024
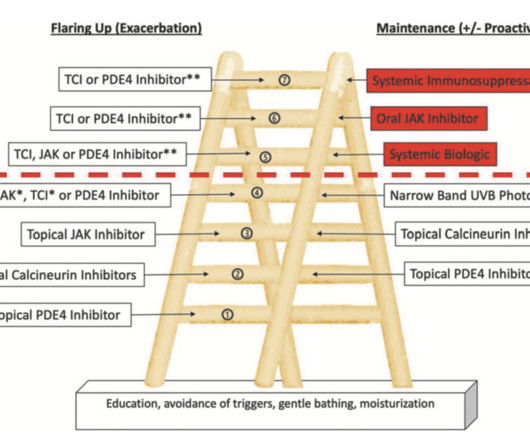
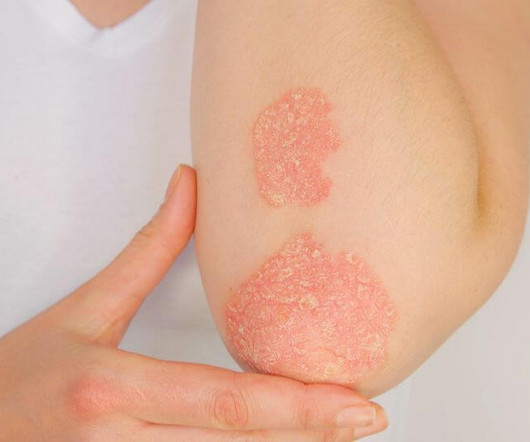
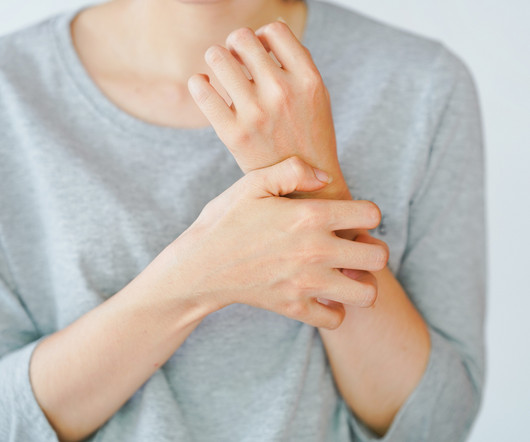
A newly developed therapeutic ladder can help guide dermatologists and patients on the use of nonsteroidal treatment options for atopic dermatitis (AD). The new topical therapies constitute the lower rungs of the therapeutic ladder and can be used for both exacerbations and maintenance. The study appears in Dermatitis.



























Let's personalize your content