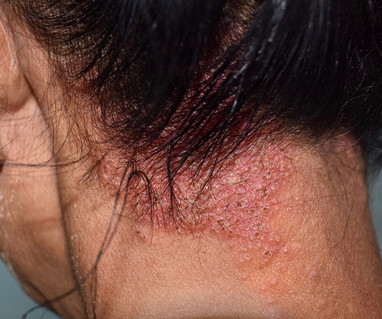
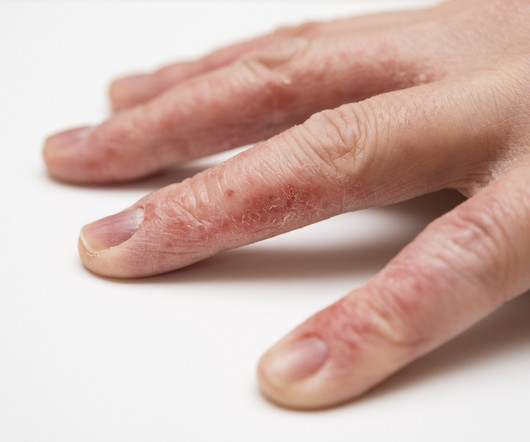
Dermavant Submits Supplemental sNDA to FDA for VTAMA Cream for AD in Adults and Children 2 Years of Age and Older
The Dermatology Digest
FEBRUARY 14, 2024
and is the same strength and formulation studied in the ADORING Phase 3 development program and included in the sNDA submission for atopic dermatitis. VTAMA cream, 1% data indicated no new safety or tolerability signals of concern in this population including children 2 years of age and older.


















Let's personalize your content