Education Event: Bridging the Gap Between Medical Professionals + Estheticians
Lipgloss and Aftershave
SEPTEMBER 3, 2024
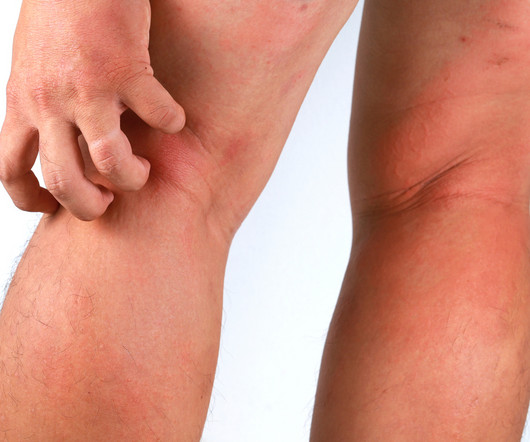
Third Annual Integrative Esthetics Conference for The Future of Skincare The third annual Integrative Esthetics Conference is a pioneering event by LearnSkin , that uniquely bridges medical professionals and estheticians, It will take place on September 29, 2024, in Arlington, Texas, and offers 7 educational topics.






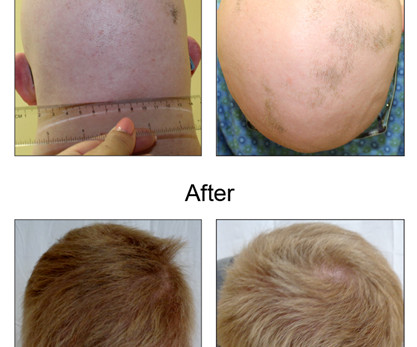

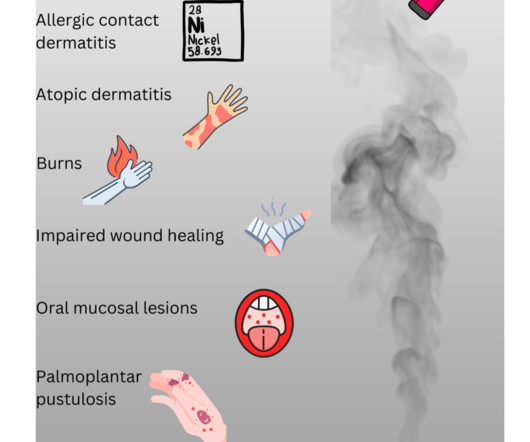


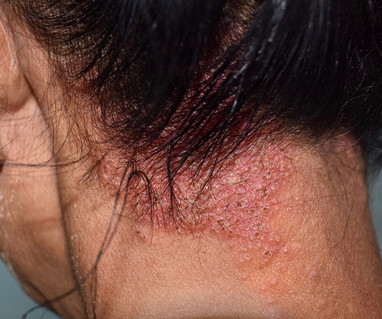




























Let's personalize your content