FLCCC I-CARE Early Treatment Protocol Review (2023)
Aesthetics Advisor
OCTOBER 7, 2023
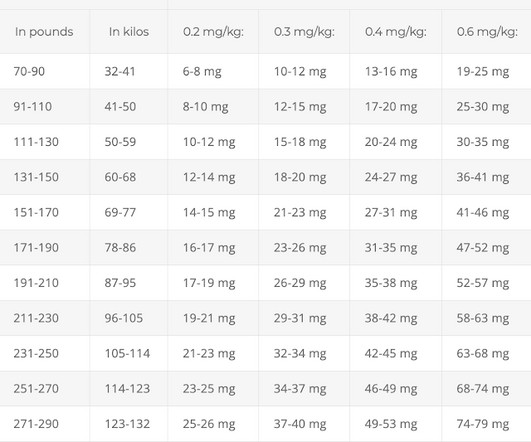
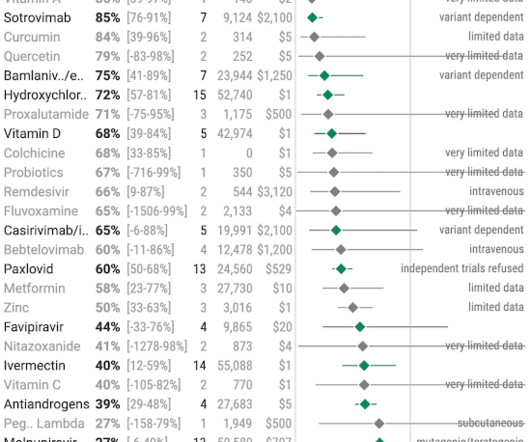
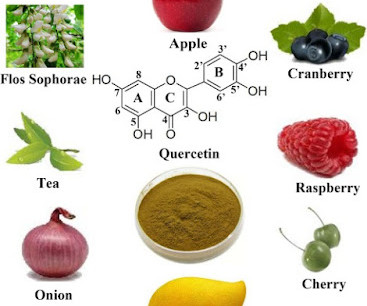
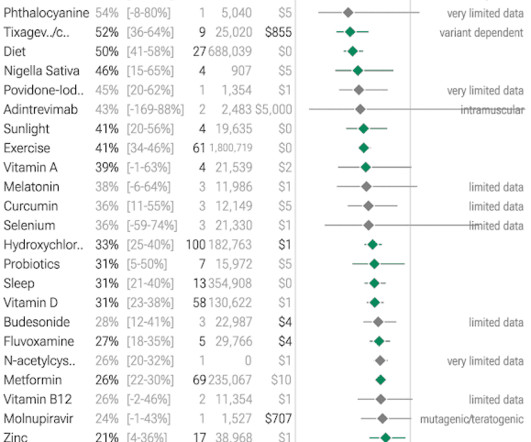
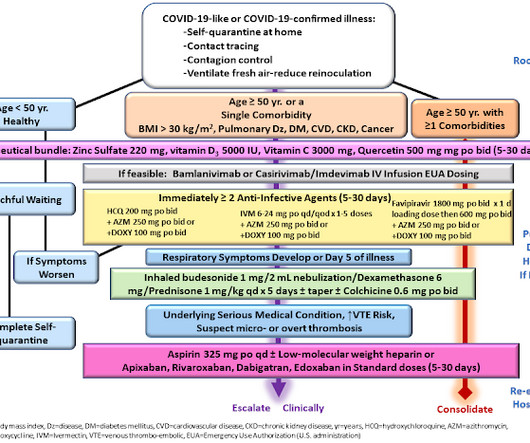
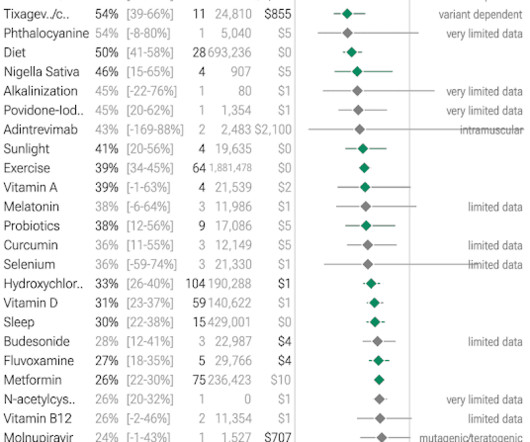
Based on rapidly emerging clinical trials evidence, the FLCCC team has developed the I-MASK+ protocol for prophylaxis and at home treatment of early stage COVID-19 and has now divided the I-MASK protocol into I-CARE and I-PREVENT protocols. Treatment is therefore highly stage-specific. Treatment of BA.4/BA.5/BQ.1.1

































Let's personalize your content