Using CO2 Laser + Other Modalities to Treat Skin Pigmentation
Lipgloss and Aftershave
NOVEMBER 1, 2024
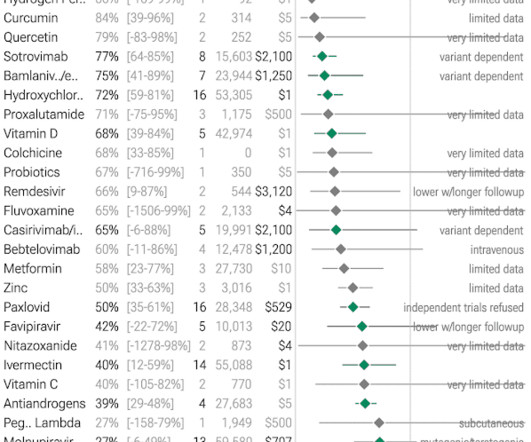
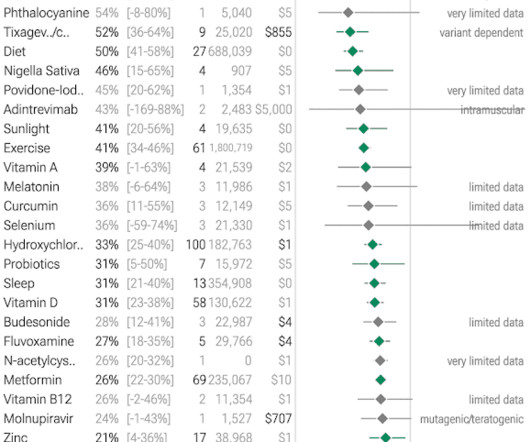
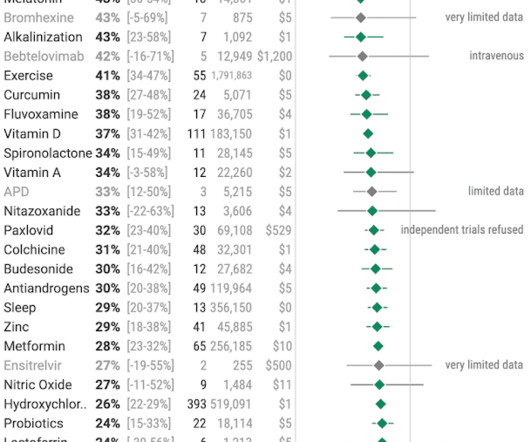
Jenni Nagle, L+A CO-Founder had a CO2 laser treatment and kept an eight day diary of her recovery process! For estheticians and skincare professionals, addressing pigmentation involves understanding and applying a multimodality treatment approach, which combines various techniques to tackle pigmentation through multiple mechanisms.
































Let's personalize your content