European Congress on Advanced Treatments and Technologies in Skin & Aesthetics
Online Laser Training
DECEMBER 8, 2019
Dr Godfrey Town gave a talk on ‘ Update on Home-Use Devices: New Devices, Regulations & Safety ’ as an Invited Speaker at the new European Congress on Advanced Treatments and Technologies in Skin & Aesthetics (Skin-Med2019) annual conference held 5th to 8th December 2019 at the Holton Munich City Hotel in Germany.






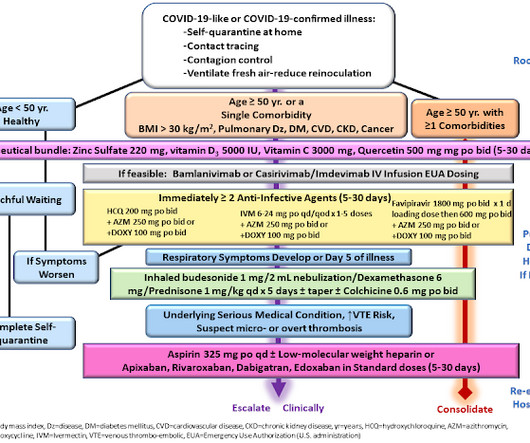


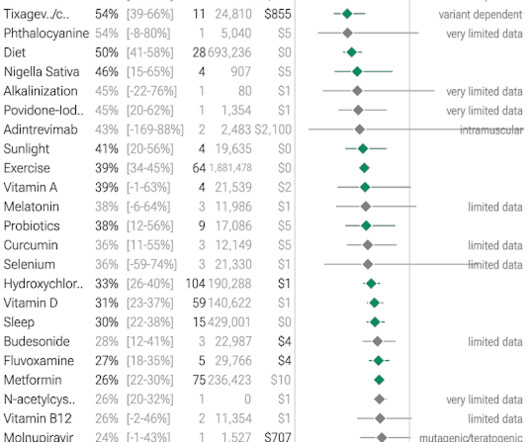

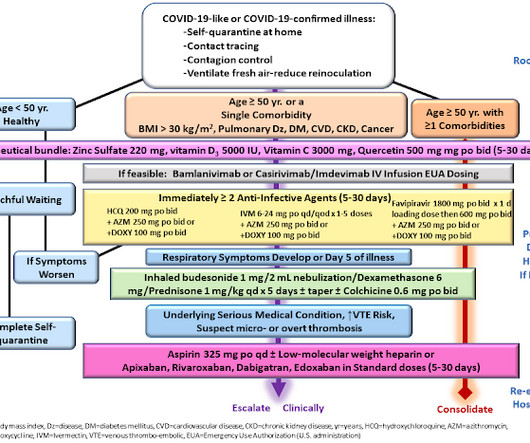
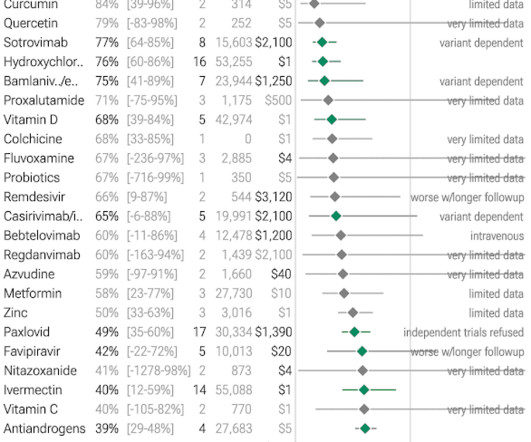










Let's personalize your content